valence electrons for as|Valences of the Chemical Elements : Tuguegarao There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h. December 11, 2014 August 13, 2018 - by Kerkay Emese. A FESZTY-KÖRKÉP TÖRTÉNETE. Kerkayné Maczky Emese. A Feszty-Körkép őseinknek a Kárpátmedencébe. való visszatérését ábrázolja. A körkép, – a panorámafestészet – külön műfaj. A mozi és a televízió elődje. Gyökerei közel 2000 évre a klasszikus Róma korába .
PH0 · What Are Valence Electrons? Definition and Periodic Table
PH1 · What Are Valence Electrons? Definition and Periodic
PH2 · Valences of the Chemical Elements
PH3 · Valence electron
PH4 · Valence Electrons Chart for All Elements
PH5 · Valence Electrons
PH6 · How to Find the Valence Electrons for Arsenic (As)
PH7 · How Many Valence Electrons Does Arsenic (As) Have?
PH8 · 3.1: Valence Electrons
PH9 · 3.10: Valence Electrons
PH10 · 10.6: Valence Electrons
In der Regel ist ein guter Lapalingo Casino Bonus nicht nur auf Merkur Automaten beschränkt, stattdessen können Sie sämtliche Online-Spiele für das Begrüßungsangebot nutzen und Gratisguthaben umsetzen. Das Starterpaket für neue Lapalingo Casino-Piloten umfasst einen 200 % Bonus und 80 Freispiele auf das Play'n .
valence electrons for as*******Mar 23, 2023
There are two ways to find the number of valence electrons in Arsenic (As). The first is to use the Periodic Table to figure out how many electrons Arsenic h.In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope.
You may assume the valences of the chemical elements—the number of electrons . In chemistry and physics, a valence electron is an electron associated with an atom that can form a chemical bond and participate in a chemical reactions. Valence electrons are outer shell electrons for main . Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called inner-shell . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .Explanation of how to count valence electrons of an element using both the electron configuration and/or the position of the element on the periodic table. Find your element on the table. Now, locate the element that you want to find the valence electrons for on the table. You can do this with its chemical symbol (the letters in each box), its atomic number (the number in .
This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell contains up to .
The chlorine atom has the same electron configuration in the valence shell, but because the entering electron is going into the n = 3 shell, it occupies a considerably larger region of space and the electron–electron repulsions are reduced. The entering electron does not experience as much repulsion and the chlorine atom accepts an additional . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called inner-shell electrons and are not involved directly in the element's reactivity, or in the formation of compounds. Lithium has a single electron in the second principal .valence electrons for asFor the main group elements, the valence electron exists only in the outermost electron shell. A valence electron can exist in the inner shell of a transition metal. An atom consisting of a closed shell of valence electrons will usually be chemically inert. A valence electron can either absorb or release energy in the form of a photon.valence electrons for as Valences of the Chemical Elements For the main group elements, the valence electron exists only in the outermost electron shell. A valence electron can exist in the inner shell of a transition metal. An atom consisting of a closed shell of valence electrons will usually be chemically inert. A valence electron can either absorb or release energy in the form of a photon.Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure \(\PageIndex{1}\) shows the Lewis symbols for the elements of the third period of the periodic table.
Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant. You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
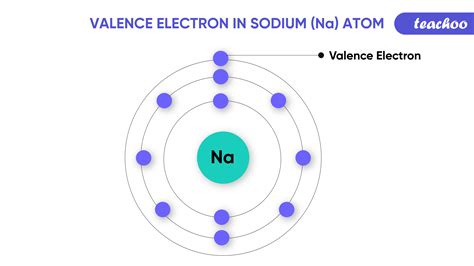
Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Valence electrons are of crucial importance because they lend deep insight into an element’s chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound – the number of bonds that . Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is a lower shell that contains an octet. 4.5: Lewis Dot and Bonding
Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd position in 3s-block. Arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. It's outermost shell (4s and 4p) has 5 electrons, these are the valence electrons. The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized (Table \(\PageIndex{1}\)). With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of . A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable electron configuration. An atom may give, take, or share electrons with another atom to achieve a full valence shell, the most stable electron configuration.

Valence Electrons. The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. Since filled d or f subshells are seldom disturbed in a chemical reaction, we can define valence electrons as follows: The electrons on an atom that are not present in the previous rare gas, ignoring filled . Valence electrons are the outermost electrons, and are the ones involved in bonding.Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons.. Electrons are arranged in "shells" or energy levels. Depending on your level of Chemistry, it is probably easier to think of them as . This electron configuration shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five. The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. The elements that receive electrons and form bonds are called anions.Valences of the Chemical Elements The valence electrons (i.e., the \(2s^22p^4\) part) are valence electrons, which do participate in the making and breaking of bonds. Similarly, in calcium (Equation \(\ref{3}\)), the electrons in the argon-like closed shell are the core electrons and the the two electrons in the 4s orbital are valence electrons.
Citi Double Cash® Card: 5 Things To Know. As you consider whether this card is right for you, here are five things I think are important for you to know. 1. The Simplicity of the Rewards Program Is Why We Like It. Clark Howard has recommended the Citi Double Cash Card as an everyday spender for years thanks to a really easy-to-understand .
valence electrons for as|Valences of the Chemical Elements